University Student Researchers Get into Battery Tech
Raising STEM career aspirations and creating a dynamic and diverse pool of talent for the fields of battery technology and energy storage.
There is currently a shortage of battery scientists and engineers to serve the growing fields of energy storage research and battery technology in the UK. This sector needs to fill 400,000 jobs if it is to meet the target of delivering net zero emissions by 2050 [1]. One of the goals of the Faraday Institution is to create a dynamic and diverse pool of talent for the sector, at every level – from school pupils, through undergraduates, to PhD students, early career researchers and beyond.
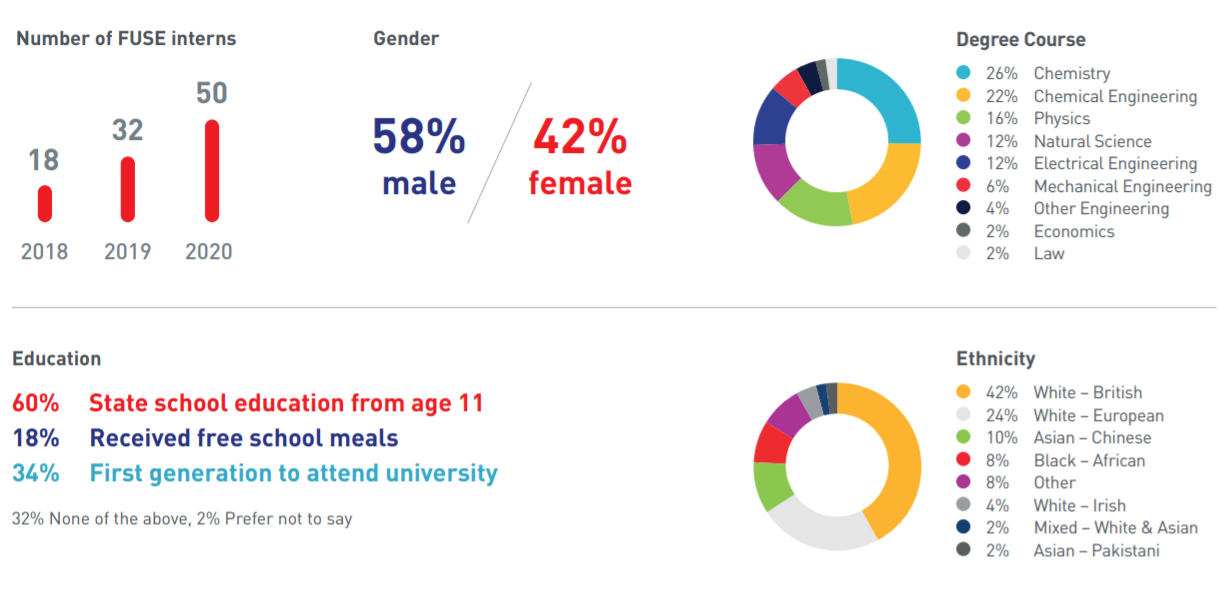
As part of its undergraduate attraction programme, each summer, the Faraday Institution funds up to 50 internships in partner universities. These 8-week, competitive internships give students access to leading scientists, unique facilities, a hands-on research experience and inspires them about future careers in STEM (science, technology, engineering and maths).
In the summer of 2020, the Faraday Undergraduate Summer Experience (FUSE) programme was adapted to create bespoke virtual internships.
In 2020, the internship programme succeeded in widening its reach and participation. A dynamic and diverse pool of talent was recruited, which included some students who had previously attended undergraduate attraction events jointly organised by SEO London and the Faraday Institution. SEO London is a charity that prepares talented students from ethnic minority or low socio-economic backgrounds for career success.
| By the Numbers | |
|---|---|
| 50 | High quality internships provided in 2020 |
| 17 | Universities involved |
| 900+ | Applications for this highly competitive programme |
| 26% | of interns (pre-placement) were considering undertaking a PhD |
| 84% | of interns (at the end of the internship) would consider a PhD |
| 93% | of respondents (at the end of the internship) would consider pursuing a career in the field of energy storage and battery technology |
Additionally, the Faraday Institution hosted a programme of career shaping events to give interns greater insight into the field. Guest speakers presented on a day in the life of a battery researcher, what a PhD in research entails, and a glimpse into launching an entrepreneurial spin-out.
Results from this programme were highlighted by the Royal Society of Chemistry: Remote Teaching and Learning During a Pandemic. Rounding out the career development aspects of the programme, interns presented scientific posters at the Faraday Institution Annual Conference (see some of the winning posters from the 2020 cohort below).
I honestly feel as though I have experienced the highs and lows of innovative research and how exciting it is.” Read more.
Ellie Bibby, FUSE intern, University of Sheffield
The guidance given for future careers was invaluable to the experience and makes this internship stand out.”
Matthew Quarrell, FUSE intern, University of Liverpool
A number of graduates of this programme have since gone on to pursue PhDs in energy storage, to further internships, to work in battery development for UK industry and to intern for a Faraday Institution spin out.
Reference
Success story published November 2020.




